Prince Sterilization Services Overview
FDA Registered • ISO 13485 Certified • cGMP
Prince is proud to serve the pharmaceutical and medical device markets with contract steam and dry heat sterilization services and support in addition to our expansive portfolio of sterile ready to use (RTU) vials and related pharmaceutical components

Take a look at Prince’s expansive SteriVial® and RTU component platform manufactured to satisfy the strict requirements of the pharmaceutical and compound pharmacy markets. All products are manufactured in accordance with cGMP and are certified sterile and RTU. All product contact components are further certified to comply with USP <85> Endotoxin and USP <788> Particulate Matter in Injections. Prince is strategically setup to process any manufacturers vials, stoppers, or seals.
Sterile Empty Vials (SEVs) – SteriVials®
Type I pharmaceutical glass vials WFI rinsed, depyrogenated, and terminally sterilized. With a foldable corrugated plastic tray and an easy-tear bag, these vials are packaged to allow for convenient and streamlined handling by the end user. Available in 2cc-100cc.
Sterile Empty Assembled Vials (SEAVs) – AssembiVials®
Type I pharmaceutical glass vials WFI rinsed, and depyrogenated assembled with a sterile stopper and seal. With a foldable corrugated plastic tray and an easy-tear bag, these vials are packaged to allow for convenient and streamlined handling by the end user. Available in 2cc-100cc.
Nest and Tub
Plug right into your flex-filling line with Prince’s patent pending RTU nest & tub vial product line. We can accommodate ANY manufacturers ISO type R vial within our ISO standard patent pending nest & tub platform. Prince’s nest & tub platform offers the end user ISO type R vials that are WFI rinsed, depyrogenated, robotically packaged into a nest and tub, and terminally sterilized, all performed in-house at Prince. Click “play” in the video to the left of this text to view this product!

Stoppers and Seals – SteriStoppers® and SteriClosures®
Pharmaceutical stoppers are WFI rinsed, packaged in 2x high quality autoclavable bags, and terminally sterilized. Aluminum seals are packaged in 2x high quality autoclavable bags and terminally sterilized. These products are certified sterile and RTU. Customize how many units you need per bag. Available in 13 and 20mm.
Contract Sterilization Services


Moist Heat Steam
Moist heat steam is the preferred sterilization method of choice amongst regulatory bodies. Prince’s steam sterilizers are cGMP and equipped to comply with EN285. Prince has expertise in the sterilization of pharmaceutical components such as bottles, stoppers, seals, glassware, tubing sets, cell culture bags and bottles, misc. GMP processing components, and medical devices. In addition, Prince has air-over-pressure sterilization capabilities and is able to terminally sterilize filled and sealed liquid pharmaceutical products within vials, ampoules, syringes, bags and other containers.
Moist Heat Steam
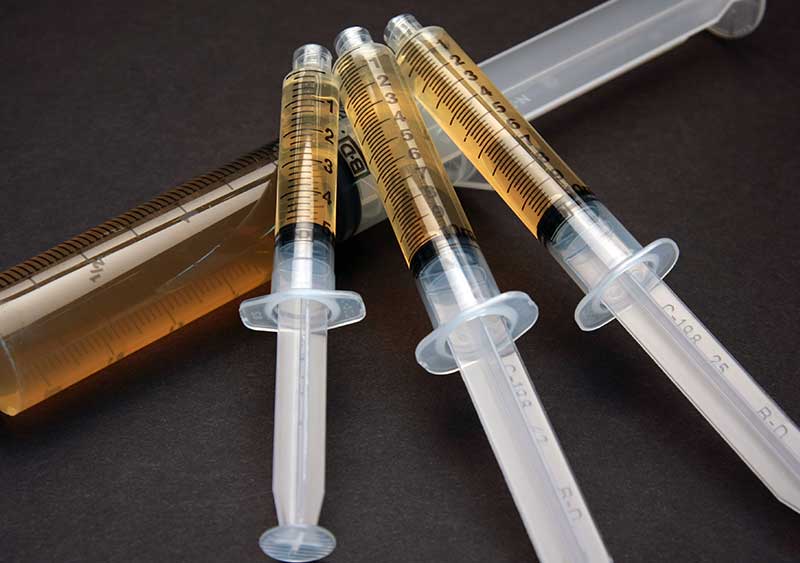

Pre-Filled Syringe Sterilization
Prince is equipped with state-of-the-art equipment specialized to provide sterilization of pre-filled syringes. Our experts can help establish sterilization cycles with parameters that achieve appropriate sterilization lethality without compromising stopper movement or syringe integrity. If you have a pre-filled syringe that requires sterilization, Prince is ready to support your efforts in development, engineering, validation, and commercial production.
Pre-Filled Syringe Sterilization


Dry Heat & Depyrogenation
Dry heat sterilization is an excellent option for components that are thermostable but sensitive to moisture. Stainless steel components such as stopper bowls and other aseptic processing equipment as well as thermostable polymers are good candidates for dry heat sterilization. Dry heat is the preferred method for depyrogenation. Glass vials, bottles, syringes, and other components that require a depyrogenation claim should be depyrogenated by heat. Prince has several dry heat ovens for batch processing as well as a dry heat tunnel for continuous washing, sterilization, and depyrogenation of pharmaceutical vials.
Dry Heat & Depyrogenation


Vaporized Hydrogen Peroxide
Vaporized Hydrogen Peroxide is an eco-friendly and non-toxic low temperature sterilization method compatible with a broad spectrum of materials. Hydrogen peroxide has excellent antimicrobial properties against a wide range of microorganisms, including bacterial spores. If you have a medical device or pharmaceutical component that needs to be sterile, hydrogen peroxide is an excellent choice.
Vaporized Hydrogen Peroxide
Benefits when you choose Prince:
- FDA Registered
- ISO 13485 certified
- cGMP
- Environmentally friendly and sustainable methods
- Nontoxic
Trust Prince to Sterilize Your Pharmaceutical or Medical Device Products
Prince offers a full suite of contract sterilization support to the pharmaceutical and medical device markets. Pre-processing support offered by Prince includes bioburden, water for injection (WFI) rinsing, packaging, sterilization, final boxing, palletization, compliance testing, and final release. Prince’s contract sterilization solutions include moist heat steam and dry heat. All of Prince’s contract sterilization solutions satisfy cGMP while also being the eco-friendliest, nontoxic, and sustainable modalities available.
Our Quality
FDA Registered – ISO 13485 Certified – cGMP – Lean Manufacturing – 5S Visual Management
Prince is committed to comply with all applicable regulatory and customer requirements to meet our customer needs in providing innovative and quality sterilization/depyrogenation services and products related to the pharmaceutical, medical device, health care, and life sciences industries. We are committed to assessing and controlling risk throughout product design and production.
Prince’s manufacturing facilities are equipped with state of the art water for injection (WFI) and purified water systems as well as industrial scale backup generators to allow for continuous operations in the event of power outage.

